Daewoong's Nabota receives positive CHMP opinion
Daewoong Pharmaceutical‘s botulinum toxin Nabota received a positive opinion from European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP), on April 26.
The CHMP opinion is a scientific recommendation for marketing authorization to the European Commission, which will now review the recommendation and deliver its final decision on the Company’s marketing authorization application. The decision will be applicable to all 28 European Union member states plus Iceland, Norway and Liechtenstein.
A positive CHMP opinion is often a sure indication of EMA approval.
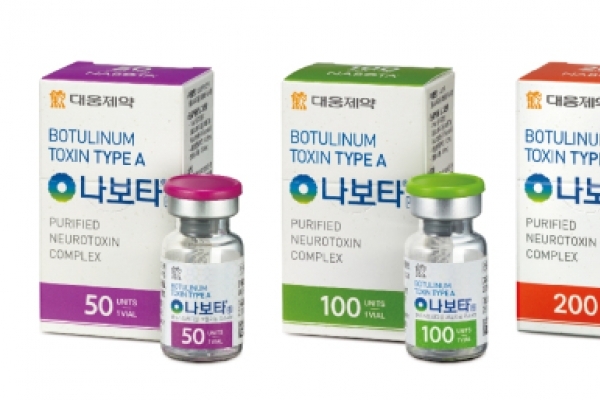
“US and Europe account for over 70 percent of the world‘s botulinum toxin market. Nabota’s entry to Europe will bolster Nabota‘s global branding,” Daewoong's spokesperson said.
Nabota, launched in Korea in 2014 by Daewoong, is marketed by Evolus in the US under the name Jeuveau. Jeuveau was approved by the US‘ Food and Drug Agency in February. In Europe, the botulinum toxin will sell under the name Nuceiva by Evolus.
By Lim Jeong-yeo/The Korea Herald (kaylalim@heraldcorp.com)
EDITOR'S PICKS
- Seoul shares rattled by Israeli attack on Iran; Kospi dips to nearly 11-week low
- S-Oil donates W560m to support firefighters
- LG CNS teams up with Yonsei University to nurture AI specialists
- Polestar 4 to make Korean debut in June
- S. Korea pledges W23tr venture capital fund for green investment at G20 meeting
- Sungsimdang outperforms bakery giants to log sales over W100b
- France rejects opening Paris flight routes to T'way Air, deals blow to Korean Air merger
- SK hynix chief underscores chip cooperation between Korea, US