YD Global Life Science files IND for diabetic retinopathy drug to begin phase 2a trials
[THE INVESTOR] YD Global Life Science said on July 17 that it filed an application with the US Food and Drug Administration for phase 2a clinical trials of its diabetic retinopathy drug candidate YD-312.
Diabetic retinopathy is the most common eye disease that affects millions of people with diabetes and a leading cause of vision loss.

“YD Global Life Science’s YD-312 has achieved an increase in potential for global market entry by nearly completing filing patent applications in major markets like the US, Europe, Japan and Canada,” CEO Lee Jin-woo said.
The drug candidate is designed to inhibit retinal vascular permeability that causes vision impairment, according to the firm.
The global diabetic retinopathy market is anticipated to reach 8.45 trillion won (US$7.52 billion) by 2020.
By Park Han-na (hnpark@heraldcorp.com)
EDITOR'S PICKS
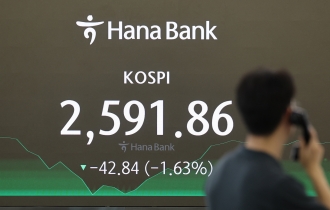
- Seoul shares rattled by Israeli attack on Iran; Kospi dips to nearly 11-week low
- S-Oil donates W560m to support firefighters
- LG CNS teams up with Yonsei University to nurture AI specialists
- Polestar 4 to make Korean debut in June
- S. Korea pledges W23tr venture capital fund for green investment at G20 meeting
- Sungsimdang outperforms bakery giants to log sales over W100b
- France rejects opening Paris flight routes to T'way Air, deals blow to Korean Air merger
- SK hynix chief underscores chip cooperation between Korea, US