Korean drugmakers gear up for launch of Gilead’s Viread generics
[THE INVESTOR] Korean drug makers are poised to challenge Gilead Sciences with their own versions of the US firm’s blockbuster treatment Viread here, industry sources said on Aug. 16.
As the Ministry of Food and Drug Safety approved 20 therapies referencing chronic hepatitis B treatment Viread earlier this month, the generic alternatives are set to be launched in November, when the original drug goes out of patent.
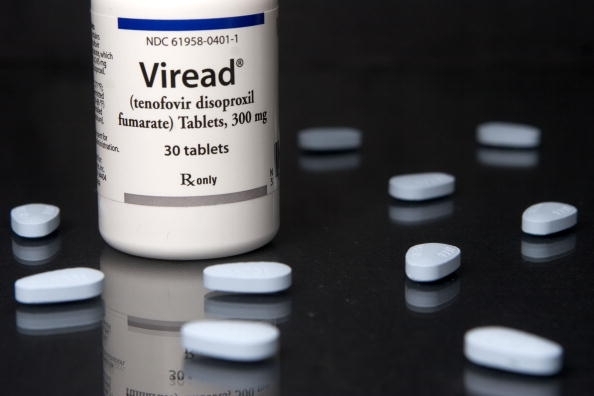
The branded drug, which is also used to prevent and treat HIV and AIDS, was the most widely prescribed therapy in Korea, raking in revenue of about 81.5 billion won (US$71.33 million) in the first six months of 2017.
The pharmaceutical companies which won approvals include JW Pharmaceutical, Chong Kun Dang Pharmaceutical, Dong-A ST, Daewoong Pharmaceutical, CJ Healthcare and Hanmi Pharmaceutical.
“We plan to introduce our version before the end of this year. Work is underway to fine tune manufacturing and marketing process,” a JW Pharmaceutical told The Investor.
To fend off impending generic competition, Gilead Sciences has developed a next-generation hepatitis B treatment, Vemlidy. The drug can be given at one-tenth the dose of Viread and has better renal and bone safety parameters than its predecessor. In Korea, Vemlidy received marketing approval in May.
By Park Han-na (hnpark@heraldcorp.com)
EDITOR'S PICKS
- Seoul shares rattled by Israeli attack on Iran; Kospi dips to nearly 11-week low
- S-Oil donates W560m to support firefighters
- LG CNS teams up with Yonsei University to nurture AI specialists
- Polestar 4 to make Korean debut in June
- S. Korea pledges W23tr venture capital fund for green investment at G20 meeting
- Sungsimdang outperforms bakery giants to log sales over W100b
- France rejects opening Paris flight routes to T'way Air, deals blow to Korean Air merger
- SK hynix chief underscores chip cooperation between Korea, US