DaeHwa Pharma seeks to launch clinical trials of oral paclitaxel in US
[THE INVESTOR] DaeHwa Pharmaceutical plans to start clinical studies of its oral formulation of cancer chemotherapy medication paclitaxel in the US, an industry source said on May 22.
“DaeHwa is planning the US clinical trials to license out DHP107 once clinical data is secured, and license negotiations with global companies become more favorable,” an official at local pharma company said.
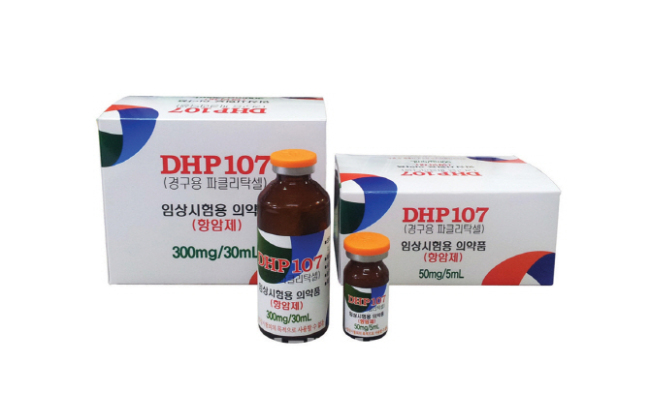
The Korean drug maker will submit an investigational new drug application to the US Food and Drug Administration to initiate phase 1 study of DHP107 for the treatment of breast cancer next month.
DaeHwa is expected to invest 5 billion won (US$4.47 million) for the early-stage studies and hopes to conduct phase 3 clinical program with a global partner that it is currently searching for.
In September last year, the drug was granted approval to treat stomach cancer, the most common type of tumor in Korea, by the country’s Ministry of Food and Drug Safety. Breast cancer is the most commonly diagnosed cancer among Americans.
DHP107 is a modified version of paclitaxel, also known as Taxol developed by Bristol-Myers Squibb, that interferes with the growth of cancer cells and slows their growth and spread in the body and is used to treat breast, lung and ovarian cancer.
Oral intake of DHP107 will improve patients convenience as paclitaxel had to be diluted before it was administered via intravenous injection. And steroids and antihistamine had to be administered to prevent possible allergic reaction, including shock and hyperventilating, to the diluents.
By Park Han-na (hnpark@heraldcorp.com)
EDITOR'S PICKS
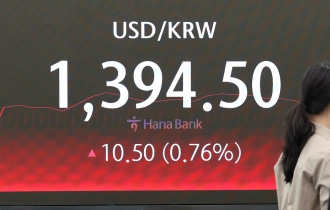
- Korean won slides amid heightened uncertainty
- Line Next redefines digital market in Web3 era
- Credit card transactions hit W900tr in 2023
- Renault Korea revisits evolution of lozenge emblem
- Finance chiefs of Korea, US, Japan to hold 3-way talks for first time
- Chanel, Louis Vuitton see muted growth in Korea
- Pineapple, mango imports surge to record highs in March
- Doosan, LG team up to debut EV charging robot